Difference between revisions of "Renewable Energy Competition"
(Ting Yu Huang 2012-08-01) |
(→Individual Lab Report: 2014-01-09 Roger Avendano) |
||
| Line 71: | Line 71: | ||
* Describe how your design succeeded or failed. What choices could you have made to improve your final standing in the competition? | * Describe how your design succeeded or failed. What choices could you have made to improve your final standing in the competition? | ||
* Discuss how you would improve the competition ratio, how was your design compared to the other group’s designs. | * Discuss how you would improve the competition ratio, how was your design compared to the other group’s designs. | ||
* Describe the power source chosen for the design. | |||
=== Team PowerPoint Presentation === | === Team PowerPoint Presentation === | ||
Revision as of 11:27, 16 January 2014
Objective
The experimental objective is to study the different types of renewable energy sources and power storage devices including hydrogen fuel cell by going through various setups provided in the lab. Design a renewable energy vehicle using the power sources and power storage devices provided in the lab to compete in a competition within the section.
Overview
Types of Renewable Energy
Renewable energy are types of energy that can be harnessed from naturally replenished resources, some examples of this includes sunlight, wind, and water. There are many benefits of using renewable energy such as clean energy sources, and they come from an abundant source that doesn’t become depleted. If we are able to efficiently utilize these renewable resources we can solve the problems with using non-renewable energy sources such as fossil fuels. [1]
Solar Power
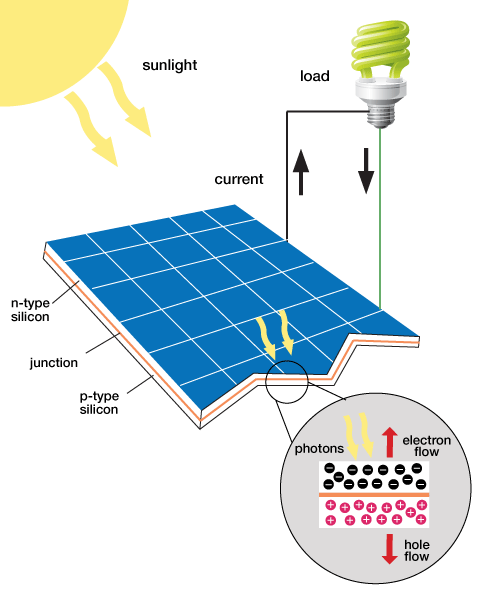
Sunlight, like any other types of light contains energy. Typically when the sunlight hits an object the energy that it contains is converted into heat. However certain materials can convert the energy into electrical current, and in this form we can harness it and store it as energy. Some solar panels are made out of large crystalline structures using the material silicon. The silicon on the solar panels would capture the free electrons from sunlight creating a potential difference between two plates. The silicon can convert a large amount of the sunlight into electrical energy but it was not cost effective because it is expensive to produce solar panels using silicon.[2]
Another common material used to create solar panels is the material Copper indium gallium (di)selenide (CIGS), which has a smaller crystalline structure but are less expensive. CIGS are relatively flexible and can easily be shaped into flexible films. The use of CIGS to make solar panels is referred to as thin-film solar technology because of its flexible nature. However CIGS are not as good at converting the absorbed light into electrical current compared to silicon, but for mass production purposes CIGS solar panels would be the more cost effective approach to produce solar panels for frequent use. [2]
In a crystal structure, the materials used for solar panels contain covalent bonds where electrons are shared by the atoms within the crystal. When the light is absorbed, electrons within the crystal become excited and move to a higher energy level. When this occurs the electron has more freedom of motion within the crystal. When the electrons move around the crystal structure an electrical current is generated, this in turn gives us electrical energy. The reaction that occurs when sunlight shines onto a solar panel is shown in Figure 1. [2]
Wind-turbine
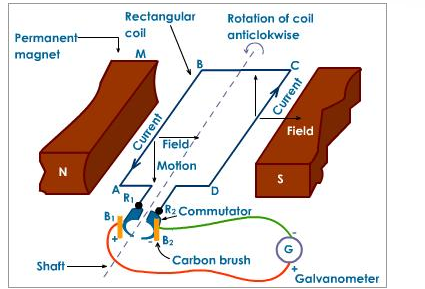
Wind-turbines are used to capture the wind’s energy and convert it into electrical energy, which can later be used to power cities. The structure of the wind-turbines has a heavy influence on its functionality. The blades on the wind-turbine are shaped so that as the wind passes through the blades it creates an uneven pressure on each side, which causes it to spin (similar to the mechanism used to keep planes airborne). As the wind-turbine spins it will cause a low speed shaft (which is connected to a gearbox) inside the blade to spin. The gearbox within the wind-turbine converts the low speed rotation to a high speed rotation through a high speed shaft. The high speed shaft is connected to a brake and then into an electrical generator, which then converts this mechanical energy into electrical energy. [3]
The electrical generator is made up of permanent magnets on each side with a rectangular coil connected to a commutator, a rotary electrical switch. The two permanent magnets on each side have creates a magnetic field. As the rectangular coil spins mechanically between the two poles of permanent magnets as shown in Figure 2, an electrical current is generated. This electrical output is then used as electrical energy. [3]
Hydrogen fuel cells
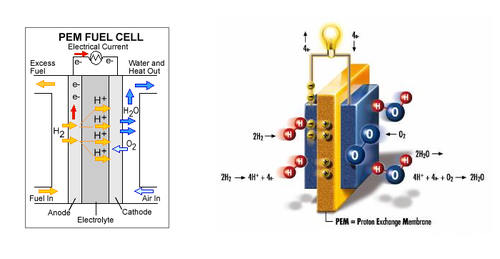
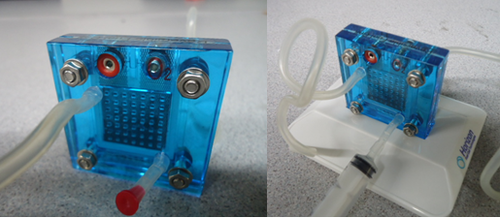
A fuel cell is a power storage device which stores chemical energy and converts it into electrical energy. The fuels uses can range from hydrogen, methane to even gasoline. For the purpose of this lab we will be using hydrogen as a fuel; therefore we are using hydrogen fuel cells. The main components that make up the fuel cell are an electrolyte, an anode chamber, a cathode chamber, and bipolar plates. The electrolyte acts as a separator that keeps the reactions from interacting with each other, by separating the anode and cathode chambers. When the water molecules are broken down, the hydrogen is stored in the anode chamber and oxygen on the cathode chamber. The bipolar plates collect the current generated by the fuel cell when the reaction takes place. Refer to Figure 3 for the internal structure of a fuel cell. [4]
Generally the gas used by the fuel cell is already separated prior to the use with a fuel cell using various methods such as using an electrolyzer, a device that separates distilled water into hydrogen and oxygen. In some cases fuel cells can be used to initiate this reaction, they are called reversible fuel cells. When supplied with water the reversible fuel cell separates the distilled water into hydrogen and oxygen. The stored hydrogen and oxygen can then be supplied to the fuel cell to generate electrical energy. Hydrogen fuel cells can be used to power various utilities such as computers, cars, power plants and even whole cities. However due to the nature of producing and storing the gas required to generate large amounts of electrical energy fuel cells are generally very large. This becomes a problem when being used in vehicles.
Electrical Components
In electrical engineering different electrical components are represented by different symbols. Below a few of them are shown:
A cell is a single unit for the conversion of chemical energy to electrical energy. A battery is comprised of multiple cells linked together.
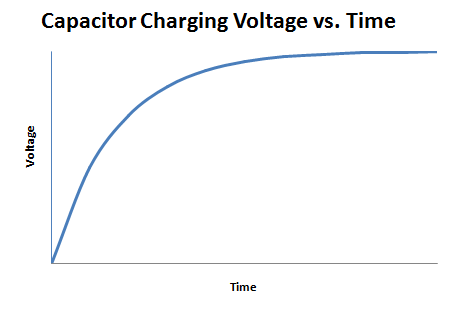
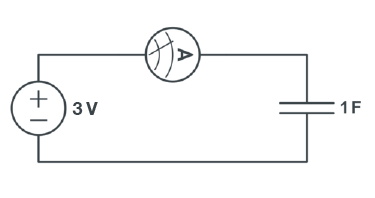
A capacitor is an electrical device used to store charge temporarily. Some capacitors can be used in place of a battery but they operate very differently from one. A capacitor is charged by a voltage source logarithmically, as shown in Figure 5.
Because of their design, these capacitors are sensitive to the polarity of the voltage applied to them. The capacitors used in this lab must be connected with the proper polarity, or they will fail. Therefore, be sure to check the capacitor used to make sure you have the negative sign on the capacitor connected to the negative applied voltage. Failure to do this will cause the capacitors to fail.
The total energy a capacitor holds is given by the equation E = CV2 / 2. Note that the energy that the capacitor holds is proportional to the square of the voltage.
A diode is a device that allows current to pass through it in only one direction. This means that it has polarity, and will only allow current to pass from its positive side to its negative side. A Light Emitting Diode (LED) not only passes current, but also lights up when it's passing current.
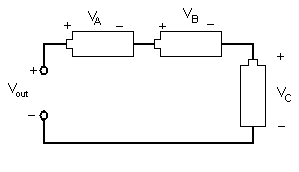
Different combinations of electrical components allow engineers to design different devices. Resistors, inductors, and capacitors can be arranged in three different ways. In a series circuit, the element's conductors are connected end to end. The current in a series circuit remains the same in all the electrical elements. In a series circuit, as shown in Figure 7, the sum of the voltages across each element is equal to the voltage of the power source (Vout = VA + VB + VC).
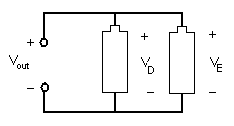
In a parallel circuit, as shown in Figure 8, the element's conductors are connected at opposing ends. The current that is supplied by the voltage source equals the current that flows though elements D and E. The voltage across the elements that are parallel is the same (Vout = VD = VE).
The motor provided is a 9V motor that will operate with voltages lower than 9V with reduced torque and speed. A motor with no load draws 9mA; a stalled motor draws well over 350mA. By increasing the voltage provided to the motor you can increase the motor speed. By increasing current you increase the torque.
Your Assignment
Individual Lab Report
Follow the lab report guidelines laid out in the page called Specifications for Writing Your Lab Reports in the Technical Communication section of this manual. As you write, the following discussion points should be addressed in the appropriate section of your lab report:
- Describe the rules of the competition in your introduction. What consequences did the rules have for your design decisions? Use the appropriate equations in your answer.
- Explain how solar panels and wind turbines work.
- Explain the concepts of the hydrogen fuel cell and capacitors.
- Discuss the advantages and disadvantages of the solar panel and wind-turbine.
- Discuss the advantages and disadvantages of hydrogen fuel cell compared to other storage devices (e.g. capacitors or batteries)
- Describe your renewable energy vehicle design and explain the choices you made in your design.
- Discuss minimal design. Did you use all the materials you purchased? Describe the importance of minimal design and explain how you employed it in your design. (how did you minimize your cost)
- Describe how your design succeeded or failed. What choices could you have made to improve your final standing in the competition?
- Discuss how you would improve the competition ratio, how was your design compared to the other group’s designs.
- Describe the power source chosen for the design.
Team PowerPoint Presentation
Follow the presentation guidelines laid out in the EG1003 Lab Presentation Format section of this manual. When you are preparing your presentation, consider the following points:
- Since one term in the competition ratio is cost, present the cost of your vehicle. Use the page How to Show Cost Data in Presentations for instructions on how to do this.
- How would you improve your renewable energy powered car?
Materials and Equipment
- Horizon Wind-Turbine
- Sunforce 50013 1-Watt Solar Battery Charger
- Adjustable Table fan
- Heat Lamp
- DMM (Digital Multi-meter)
- Music Voltmeter
- 2V DC Motor
- Horizon Hydrogen Fuel cell
- 1 Farad 2.5V Capacitor
- Mini Electric propeller
- LED (Light Emitting Diode)
- 3 Alligator cable sets
- Standard Lego Car Chassis plus Lego parts kit
- Lego to Alligator Cable Clip Connector
- Scissors
- Tape
Procedure
Part 1: Testing the Power sources
Wind turbine
- Connect the Wind turbine to the digital multi-meter using the alligator clips provided. The connection is shown in Figure 9 below. Place the wind turbine in front of the fan and take down the voltage.
- Disconnect the two connections from the digital multi-meter and connect the alligator clips to the Music Voltmeter. Place the wind turbine in front of the fan and observe what happens to the music voltmeter.
Solar Panel
- Connect the solar panel to the digital multi-meter using the alligator clips (refer to Figure 10 below). Place the solar panel near the window or a heat lamp and record the voltage reading.
- Repeat the process in step two and observe the results from Music Voltmeter.
- Caution! The heat lamps and solar panels may become extremely hot when used for a long duration of time do not touch them immediately after use and turn them off when not in use.
Part 2: Testing the Power storage devices
Preparing the Hydrogen Fuel cell
- Place the 2x4cm (small) length pieces of rubber tube into the bottom right pin on the hydrogen fuel cell (on both sides) and insert a cap (red for oxygen and black for hydrogen) into the other end of the tube.
- Insert the two longer tubes into the top left pin of the hydrogen fuel cell.
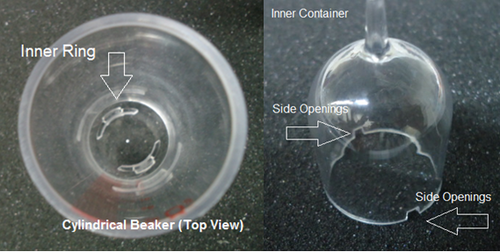
- Take the two cylindrical beakers and place them on top of the stand provided locking it in place, then fill the two beakers with distilled water until the water level reaches zero.
- Place the two inner containers into the cylindrical beakers (make sure the side openings on the inner container is not blocked by the inner ring on the beakers), the distilled water from the beaker will fill out the inner container at this point.
- On the oxygen side of the fuel cell connect the syringe to the uncapped tube (first remove the red cap on the smaller tube). Fill the reversible fuel cell with distilled water using the syringe until all the air is removed from the long tube. Afterwards place the red cap on the smaller tube
- IMPORTANT: Make sure the connections of the wires to the fuel cell match (red to red, black to black) otherwise it may lead to failure of this part of the experiment and damage to the fuel cell. If this problem occurs the students will have to take full responsibility for replacing the parts.
- Hint: Place the longer tube above the oxygen beaker to hold the water coming out of the longer tube.
- Connect the two longer tubes to the inner container on each of the beakers corresponding to oxygen and hydrogen on the fuel cell. (Make sure there is no air in the inner containers.)
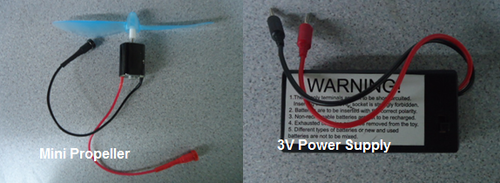
- Connect the 3V power supply to the positive and negative inputs on the fuel cell and turn on the power supply.
- Charge until both containers (oxygen and hydrogen) are filled with gas.
- Discharge the charged fuel cell by connecting it to the mini propeller provided.
Charging a capacitor
- Connect an alligator clip to each of the cables coming out of the 3V power supply. (red is positive and black is negative)
- Connect to the one of unconnected alligator clips the ammeter (multimeter setting)
- Connect the rest of the unconnected cables (one from ammeter and one alligator clip) connect to pins of the capacitor with correspond polarity. (refer to Figure 10)
- Charge until the current is zero.
- Discharge the charged capacitor by connecting it to the mini propeller provided.
Part 3: Renewable Car competition
- Assess your materials and consider the data established from Part 1 and Part 2. Choose materials for your car design, keeping in mind the Competition Ratio. Make sure you take notes and make preliminary sketches during this process. (You may choose different combinations of power sources and power storage devices for your design)
- You will be given a standard chassis for your renewable energy vehicle. Your design must be able to hold the power storage devices on top of it during the trials (you may modify your design to fit the power storage devices as you deem fit). Prepare a price-list for your renewable energy powered vehicle based on the setups and materials you have chosen. Have your TA sign the sketches and the price-list.
- Your TA will provide the materials needed for your design. If you decide to modify your design during the construction of your car, note the changes and describe the reasons for them. If the modifications required more materials to be used, make sure you update your price list and your TA approves it. (Note: You may modify your design between trials)
Before entering the competition, test if your motor is working. This can be done by using the lighting system from your LabVIEW lab. Attach the red alligator clip to one end of an LED. Attach the black alligator clip to the other end of the LED. Run the LabVIEW program. The motor should run when the LED that connects the motor lights up. If the motor doesn't run, ask your TA for another motor.
Competition
The renewable energy vehicle must be able to carry the power storage device you choose to use on it (e.g. fuel cell, capacitor). You may only power your capacitor and fuel cell using the power sources provided, violations to these rules will result in a failing grade for the lab report for this lab.
When requested by the TA, the student will position the renewable energy car and complete the necessary connections before the trial begins.
The competition will be won by the team that has the highest competition ratio:
The TA will record the test data after five minutes or when the car stops moving, whichever occurs first. The TA will then calculate the competition ratio. The tabulation for the whole class will be provided.
References
[1] NextEra Energy Resources, LLC., . "Benefits of Renewable Energy." NextEra Energy Resources. NextEra Energy Resources, 2012. Web. 24 Jul 2012. <http://www.nexteraenergyresources.com/content/environment/benefits.shtml>.
[2] Locke, S.. "How does solar power work." Scientific american. Scientific American, 2008. Web. 24 Jul 2012. <http://www.scientificamerican.com/article.cfm?id=how-does-solar-power-work>.
[3] Layton, J.. "How Wind Power Works." How stuff works. Discovery, 2011. Web. 24 Jul 2012. <http://science.howstuffworks.com/environmental/green-science/wind-power.htm>.
[4] Reg Tyler, . "Types of Fuel Cells." Energy efficeny and renewable energy. U.S. Department of Energy, 2011. Web. 24 Jul 2012. <http://www1.eere.energy.gov/hydrogenandfuelcells/fuelcells/fc_types.html>.
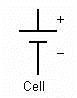





![{\displaystyle CR={\frac {distance\left[{\text{ft}}\right]}{1\left[{\text{s}}\right]+time\left[{\text{s}}\right]}}\times {\frac {100}{Cost\left[\$\right]}}+distance\left[{\text{ft}}\right]\,}](https://wikimedia.org/api/rest_v1/media/math/render/png/a0643569de9df32ae019a6925b9b94366fc82c0e)