Renewable Energy Competition
Objective
The experimental objective is to evaluate different sources of renewable energy and design a renewable energy vehicle using the power sources and power storage devices provided in the lab to compete in a competition in the section.
Overview
Types of Renewable Energy
Renewable energy sources are types of energy that can be harnessed from naturally replenished resources. Some examples include sunlight, wind, and water. There are many benefits to using renewable energy. They are clean energy sources, and they come from an abundant source that does not become depleted. If these sources can be utilized efficiently, this can solve the problems with using non-renewable energy sources, such as fossil fuels (NextEra Energy Resources, LLC, 2012).
Solar Power
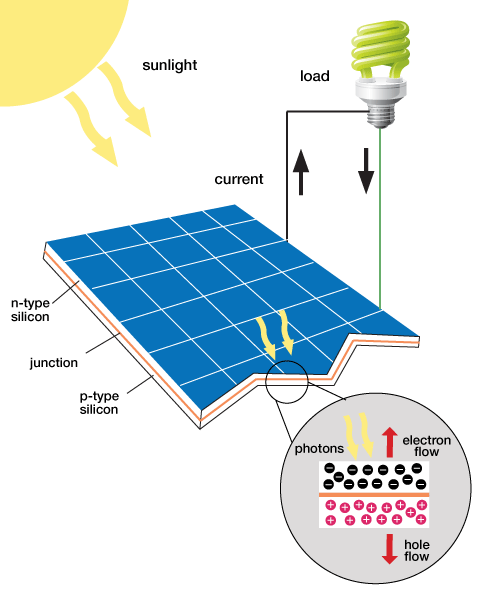
Sunlight, like any other type of light, contains energy. Typically when the sunlight hits an object, the energy it contains is converted into heat. Certain materials can convert the energy into electrical current that can be harnessed and stored as energy. Some solar panels are made out of large crystalline structures using the material silicon. The silicon on the solar panels captures the free electrons from sunlight creating a potential difference between two plates. The silicon can convert a large amount of the sunlight into electrical energy, but it is not cost effective because it is expensive to produce solar panels using silicon (Locke, 2012).
Another common material used to create solar panels is Copper indium gallium (di)selenide (CIGS), which has a smaller crystalline structure, but is less expensive. CIGS is relatively flexible and can easily be shaped into flexible films. The use of CIGS to make solar panels is referred to as thin-film solar technology because of its flexible nature. CIGS is not as good at converting the absorbed light into electrical current compared to silicon, but for mass production purposes, CIGS solar panels are the more cost effective approach to produce solar panels for frequent use (Locke, 2012).
The materials used for solar panels contain covalent bonds where electrons are shared by the atoms within the crystal. When the light is absorbed, electrons within the crystal become excited and move to a higher energy level. When this occurs, the electron has more freedom of motion within the crystal. When the electrons move around the crystal structure, an electrical current is generated that produces electrical energy. The reaction that occurs when sunlight shines onto a solar panel is shown in Figure 1 (Locke, 2012).
Wind-turbine
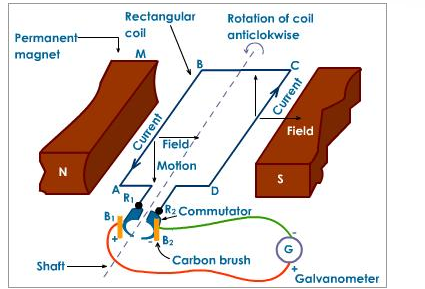
Wind-turbines are used to capture the wind’s energy and convert it into electrical energy that can later be used to power cities. The structure of the wind-turbines influences its functionality. The blades on the wind-turbine are shaped so that as the wind passes through the blades it creates an uneven pressure on each side that causes it to spin (similar to the mechanism used to keep planes airborne). As the wind-turbine spins, it causes a low speed shaft, which is connected to a gearbox, inside the blade to spin. The gearbox within the wind-turbine converts the low speed rotation to a high speed rotation through a high speed shaft. The high speed shaft is connected to a brake and then to an electrical generator that converts this mechanical energy into electrical energy (Layton, 2012).
The electrical generator is made up of permanent magnets on each side with a rectangular coil connected to a commutator, which is a rotary electrical switch. The two permanent magnets on each side create a magnetic field. As the rectangular coil spins between the two poles of permanent magnets, as shown in Figure 2, an electrical current is generated. This electrical output is then used as electrical energy (Layton, 2012).
Hydrogen fuel cells
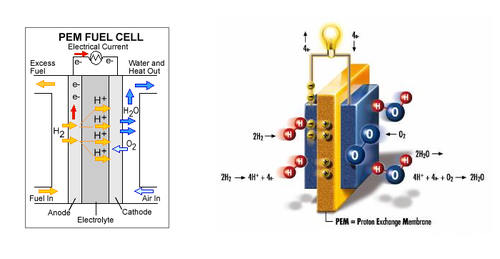
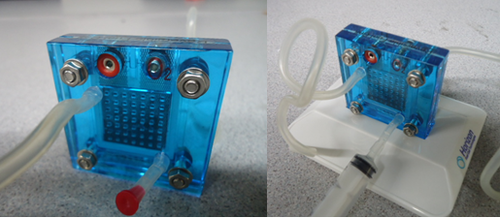
A fuel cell is a power storage device that stores chemical energy and converts it into electrical energy. The fuels used range from hydrogen, methane to gasoline. For the purpose of this lab, hydrogen will be used as a fuel so these are hydrogen fuel cells. The main components that make up the fuel cell are an electrolyte, an anode chamber, a cathode chamber, and bipolar plates. The electrolyte acts as a separator that keeps the reactions from interacting with each other by separating the anode and cathode chambers. When the water molecules are broken down, the hydrogen is stored in the anode chamber and oxygen in the cathode chamber. The bipolar plates collect the current generated by the fuel cell when the reaction takes place. Figure 3 shows the internal structure of a fuel cell (Tyler, 2012).
Generally, the gas used by a fuel cell is separated prior to use with a fuel cell using various separation methods, such as using an electrolyzer, which is a device that separates distilled water into hydrogen and oxygen. In some cases, fuel cells can be used to initiate this reaction. These fuel cells are called reversible fuel cells. When supplied with water, the reversible fuel cell separates the distilled water into hydrogen and oxygen. The stored hydrogen and oxygen can then be supplied to the fuel cell to generate electrical energy. Hydrogen fuel cells can be used to power computers, cars, power plants, and even whole cities. Due to the nature of producing and storing the gas required to generate large amounts of electrical energy, fuel cells are generally very large. This becomes a problem when used in vehicles.
Electrical Components
In electrical engineering, different electrical components are represented by different symbols. Figures 4a and 4b show two symbols.
A cell is a single unit for the conversion of chemical energy to electrical energy. A battery is comprised of multiple cells linked together.
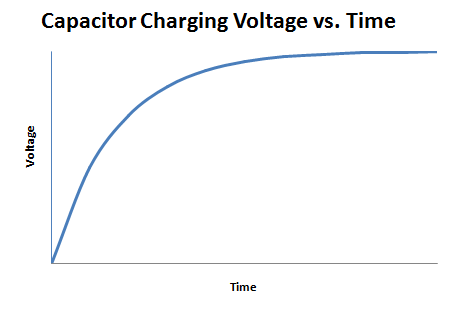
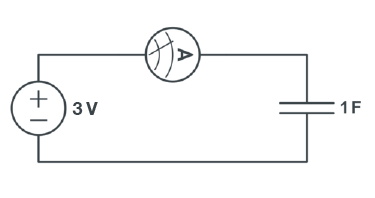
A capacitor is an electrical device that is used to store charge temporarily. Some capacitors can be used in place of a battery, but they operate very differently from a battery. A capacitor is charged by a voltage source logarithmically, as shown in Figure 5.
Because of their design, capacitors are sensitive to the polarity of the voltage applied to them. The capacitors used in this lab must be connected with the proper polarity or they will fail. Be sure to check the capacitor used to make sure the negative sign on the capacitor is connected to the negative applied voltage. Failure to do this will cause the capacitors to fail.
The total energy a capacitor holds is given by the equation E = CV2 / 2. Note that the energy holds is proportional to the square of the voltage.
A diode is a device that allows current to pass through it in only one direction. This means that it has polarity and will only allow current to pass from its positive side to its negative side. A light emitting diode (LED) passes current and lights up when it is passing current.
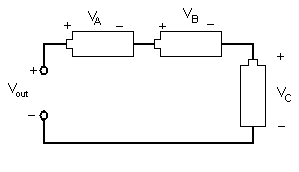
Different combinations of electrical components allow engineers to design different devices. Resistors, inductors, and capacitors can be arranged in three different ways. In a series circuit, the elements are connected end to end. The current in a series circuit remains the same in all the electrical elements. In a series circuit, as shown in Figure 7, the sum of the voltages across each element is equal to the voltage of the power source (Vout = VA + VB + VC).
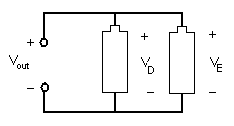
In a parallel circuit, as shown in Figure 8, the elements are connected at opposing ends. The current that is supplied by the voltage source equals the current that flows though elements D and E. The voltage across the elements that are parallel is the same (Vout = VD = VE).
The motor provided is a 9V motor that will operate with voltages lower than 9V, but with reduced torque and speed. A motor with no load draws 9mA; a stalled motor draws well over 350mA. By increasing the voltage provided to the motor, the motor speed will be increased. By increasing current, the torque is increased.
Design Considerations
- Which source yields the most voltage per unit cost?
- Which circuit configuration will provide the most desirable voltage across the load? Parallel or series?
- Which aspects of the competition formula are most advantageous?
Materials and Equipment
Materials with Price List
- A Horizon wind-turbine: $5.00 each
- Solar battery panels: $10.00 each
- A Horizon hydrogen fuel cell: $12.00 each
- A 1 Farad 2.5V capacitor: $3.00 each
- Alligator cable sets: $0.50/pair
- A standard Lego car chassis plus Lego parts kit: $0, limit one per group
- A Lego to alligator cable clip connector: $0.10 each
- Tape: $0.10/feet
Equipment Used
- A Horizon Wind-Turbine
- A Sunforce 50013 1-Watt solar battery charger
- An adjustable Table fan
- A heat lamp
- A DMM (Digital Multi-meter)
- A Music voltmeter
- A 2V DC motor
- A Horizon hydrogen fuel cell
- A 1 Farad 2.5V capacitor
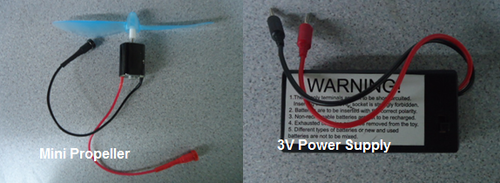
- A mini electric propeller
- An LED (Light Emitting Diode)
- 3 Alligator cable sets
- A standard Lego car chassis plus Lego parts kit
- A Lego to alligator cable clip connector
- Scissors
- Tape
Procedure
Part 1: Testing the power sources
Wind turbine
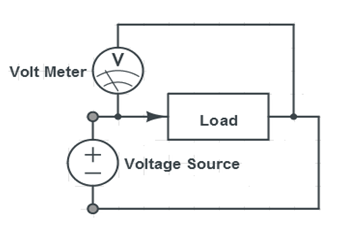
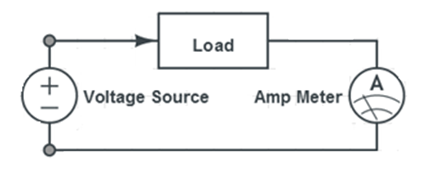
- Connect the wind turbine to the mini propeller using the alligator clips provided. Place the wind turbine in front of the fan and measure the voltage and current across the mini propeller using the DMM provided. The connections for measuring voltage and current across the mini propeller are shown in Figures 9 and 10.
- Adjust the position of the turbine to find the highest voltage and current that can be generated.
- Calculate and record the power generated by the turbine.
Solar panel
- Connect the solar panel to the mini propeller using the alligator clips provided. Place the solar panel near the heat lamp and measure the voltage and current across the mini propeller using the DMM provided. The connections for measuring voltage and current across the mini propeller are shown in Figures 9 and 10.
- Adjust the position of the solar panel to find the highest voltage and current that can be generated.
- Calculate and record the power generated by the solar panel.
- Caution! The heat lamps and solar panels may become extremely hot when used for a long duration of time. Do not touch them immediately after use and turn them off when not in use.
Part 2: Testing the power storage devices
Preparing the hydrogen fuel cell
- Place the 2x4cm (small) length pieces of rubber tube into the bottom right pin on the hydrogen fuel cell (on both sides) and insert a cap (red for oxygen and black for hydrogen) into the other end of the tube.
- Insert the two longer tubes into the top left pin of the hydrogen fuel cell (Figure 11).
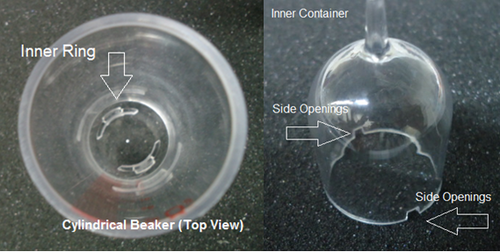
- Take the two cylindrical beakers and place them on top of the stand provided locking it in place then fill the two beakers with distilled water until the water level reaches zero (Figure 12).
- 1. Place the two inner containers into the cylindrical beakers. Make sure the side openings on the inner container are not blocked by the inner ring on the beakers (Figure 13). The distilled water from the beaker will fill out the inner container at this point.
- Connect the two longer tubes to the inner container on each of the beakers corresponding to oxygen and hydrogen on the fuel cell. Make sure there is no air in the inner containers.
- On the oxygen side of the fuel cell (Figure 14), connect the syringe to the uncapped tube (first remove the red cap on the smaller tube). Fill the reversible fuel cell with distilled water using the syringe until all the air is removed from the long tube (Figure 15). Afterwards place the red cap on the smaller tube.
- IMPORTANT: Make sure the connections of the wires to the fuel cell match (red to red, black to black) otherwise it may lead to failure of this part of the experiment and damage to the fuel cell. If this problem occurs the students will have to take full responsibility for replacing the parts.
- Hint: Place the longer tube above the oxygen beaker to hold the water coming out of the longer tube.
- Connect the 3V power supply to the positive and negative inputs on the fuel cell and turn on the power supply.
- Charge until the H2 inner container is filled with gas. The O2 should be half because the fuel cell is converting H2O and the stoichiometric coefficients demand that balance.
- Discharge the charged fuel cell by connecting it to the mini propeller provided (Figure 16).
- Measure the voltage and current across the mini propeller using the DMM provided. The connections for measuring voltage and current across the mini propeller are shown in Figures 9 and 10.
- Calculate and record the power produced by the hydrogen fuel cell.
Charging a capacitor
- Connect an alligator clip to each of the cables coming out of the 3V power supply. (red is positive and black is negative).
- Connect one of unconnected alligator clips to the ammeter (multimeter setting).
- Connect the rest of the unconnected cables (one from ammeter and one alligator clip) connect to pins of the capacitor with correspond polarity (Figure 10).
- Charge until the current is zero.
- Discharge the charged capacitor by connecting it to the mini propeller provided.
Part 3: Renewable car competition
- Assess the materials and consider the data produced from Part 1 and Part 2. Choose materials for the car design, keeping in mind the competition ratio. Create preliminary sketches during this process. Different combinations of power sources and power storage devices may be used for the design.
- The design must be able to hold the power storage devices on top of it during the trials. The design can be modified. Prepare a price-list for the renewable energy-powered vehicle based on the design and materials chosen. Have a TA sign the sketches and the price-list.
- A TA will provide the materials needed for the design. If the design is modified during construction, note the changes and describe the reasons for them. If the modifications required more materials, make sure the price list is updated and a TA approves it. Designs may be modified between trials.
Before entering the competition, test the motor.
Competition
The renewable energy vehicle must be able to carry its power storage device (e.g. fuel cell, capacitor). If a capacitor is used, it may only be powered using the power sources provided. Violations to these rules will result in a failing grade for the lab report for this lab.
When requested by the TA, the renewable energy car will be positioned and the necessary connections will be completed before the trial begins.
The design that has the highest competition ratio will win the competition (Equation 1).
Equation 1: Competition ratio.
The TA will record the test data after five minutes or when the car stops moving, whichever occurs first. The TA will then calculate the competition ratio. The tabulation for the whole class will be provided.
Assignment
Individual Lab Report
Follow the lab report guidelines laid out in Specifications for Writing Lab Reports in the Technical Communication section of this manual. The following points should be addressed in the appropriate section of the lab report:
- Describe the rules of the competition in your introduction. What consequences did the rules have for design decisions? Use the appropriate equations in the answer
- Explain how solar panels and wind turbines work
- Explain the concepts of the hydrogen fuel cell and capacitors
- Discuss the advantages and disadvantages of the solar panel and wind-turbine
- Discuss the advantages and disadvantages of hydrogen fuel cell compared to other storage devices (e.g. capacitors or batteries)
- Describe the renewable energy vehicle design and explain the design choices
- Discuss minimal design. Were all the materials purchased used? Describe the importance of minimal design and explain how it was employed in the design
- Describe how the design succeeded or failed. What choices would be made to improve its final standing in the competition?
- Discuss how to improve the competition ratio and compare the ratios
- Describe the power source chosen for the design
- Include spreadsheet with every designs’ results. Describe the results and talk about other designs in the class
Remember: Lab notes must be taken. Experimental details are easily forgotten unless written down. EG1004 Lab Notes Paper can be downloaded and printed from the EG1004 Website. Use the lab notes to write the Procedure section of the lab report. At the end of each lab, a TA will scan the lab notes and upload them to the Lab Documents section of the EG1004 Website. One point of extra credit is awarded if the lab notes are attached at the end of the lab report. Keeping careful notes is an essential component of all scientific practice.
Team PowerPoint Presentation
Follow the presentation guidelines laid out in the EG1004 Lab Presentation Format section of this manual. When preparing the presentation, consider the following points:
- Since one term in the competition ratio is cost, present the cost of the vehicle. Use the page How to Show Cost Data in Presentations for instructions on how to do this
- How can the performance of the renewable energy powered car be improved?
References
NextEra Energy Resources, LLC., . "Benefits of Renewable Energy." NextEra Energy Resources. NextEra Energy Resources, 2012. Web. 24 Jul 2012. <http://www.nexteraenergyresources.com/content/environment/benefits.shtml>.
Locke, S.. "How does solar power work." Scientific american. Scientific American, 2008. Web. 24 Jul 2012. <http://www.scientificamerican.com/article.cfm?id=how-does-solar-power-work>.
Layton, J.. "How Wind Power Works." How stuff works. Discovery, 2011. Web. 24 Jul 2012. <http://science.howstuffworks.com/environmental/green-science/wind-power.htm>.
Reg Tyler, . "Types of Fuel Cells." Energy efficeny and renewable energy. U.S. Department of Energy, 2011. Web. 24 Jul 2012. <http://www1.eere.energy.gov/hydrogenandfuelcells/fuelcells/fc_types.html>.
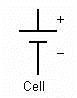



![{\displaystyle CR={\frac {distance\left[{\text{ft}}\right]}{1\left[{\text{s}}\right]+time\left[{\text{s}}\right]}}\times {\frac {100}{Cost\left[\$\right]}}+distance\left[{\text{ft}}\right]\,}](https://wikimedia.org/api/rest_v1/media/math/render/png/a0643569de9df32ae019a6925b9b94366fc82c0e) Equation 1: Competition ratio.
Equation 1: Competition ratio.