Difference between revisions of "Hot Air Balloon Competition"
Jbringardner (talk | contribs) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
= Objectives = | = Objectives = | ||
The objective of this lab is to design and build a hot air balloon. This is a competition lab that will be judged by a ratio that uses time afloat, cost, and payload. In theory, the design should maximize the non-structural weight (payload) that the balloon can lift and the time it can spend aloft while minimizing the balloon's structural weight and its cost. In practice, other design choices could also win the competition. Consider the components of the ratio and the rules before designing the balloon. | The objective of this lab is to design and build a hot air balloon. This is a competition lab that will be judged by a ratio that uses time afloat, cost, and payload. In theory, the design should maximize the non-structural weight (payload) that the balloon can lift and the time it can spend aloft while minimizing the balloon's structural weight and its cost. In practice, other design choices could also win the competition. Consider the components of the ratio and the rules before designing the balloon. | ||
| Line 91: | Line 88: | ||
Construct a hot air balloon using the available materials. This lab is a competition. The design with the highest ratio of payload divided by cost multiplied by time afloat will be the winner. | Construct a hot air balloon using the available materials. This lab is a competition. The design with the highest ratio of payload divided by cost multiplied by time afloat will be the winner. | ||
Sketch a preliminary design. The maximum balloon volume is 1 m<sup>3</sup>. Volume should be approximated. | Sketch a preliminary design. The maximum balloon volume is 1 m<sup>3</sup>. Volume should be approximated and recorded on your lab notes. | ||
The design must include an area near the bottom of the balloon where paperclips may be attached to add payload during the competition phase of the lab. In addition, there must be an opening that will allow hot air to enter the balloon when placed over the heater. | The design must include an area near the bottom of the balloon where paperclips may be attached to add payload during the competition phase of the lab. In addition, there must be an opening that will allow hot air to enter the balloon when placed over the heater. | ||
| Line 105: | Line 102: | ||
== Individual Lab Report == | == Individual Lab Report == | ||
<!-- | <!-- | ||
:''For | :''For EG1004 sections, this report is '''optional''' and will count as a Bonus Lab Report if submitted. Extra credit will be applied to the PowerPoint presentation since the report is optional.'' | ||
:''For EGED sections, this report is '''required''' and will count as a regular lab report.'' | :''For EGED sections, this report is '''required''' and will count as a regular lab report.'' | ||
--> | --> | ||
| Line 135: | Line 132: | ||
{{Reflist}} | {{Reflist}} | ||
{{Laboratory Experiments}} | <!--{{Laboratory Experiments}}--> | ||
Latest revision as of 21:39, 5 September 2022
Objectives
The objective of this lab is to design and build a hot air balloon. This is a competition lab that will be judged by a ratio that uses time afloat, cost, and payload. In theory, the design should maximize the non-structural weight (payload) that the balloon can lift and the time it can spend aloft while minimizing the balloon's structural weight and its cost. In practice, other design choices could also win the competition. Consider the components of the ratio and the rules before designing the balloon.
Overview
Hot air balloons are lighter-than-air aircrafts. They are widely used for recreation and advertising. Another example of this type of aircraft is the dirigible, which consists of a rigid steel frame with bags of light gas inside it that cause lift. Because of the weight of the frame in early dirigibles, an extremely light though flammable gas, such as hydrogen, was used. This led to safety problems, so dirigibles are no longer in service. A blimp is a lighter-than-air aircraft that is essentially a big helium balloon with engines attached. It does not have a structural frame like a dirigible. An aerostat is a blimp that does not have engines, but is attached to the ground. Aerostats are frequently used for advertising or finding a location. Aerostats are also used as weather stations and radar platforms since they can reach altitudes of up to 15,000 feet.
The NASA Balloon Program has been using high altitude scientific balloons as a platform for space and Earth science discoveries and technological innovation. A new design competition has launched to promote the research and development of better scientific balloons. The goal is to design the optimal balloon for maximizing the payload of scientific equipment it can carry and the time the balloon can remain in the air.
The Ideal Gas Law, gas density, the Principle of Archimedes, and Newton's Second Law of Motion explain why hot air balloons float.
Ideal Gas Law and Gas Density
Usually, when a gas is heated, it expands so the same mass has a larger volume and the density decreases. The expansion can be quite pronounced and a mass of warm air can be considerably less dense than an equal mass of cold air. This can be expressed quantitatively by using the Ideal Gas Law to describe the behavior of a gas. This law states:
P is the gas pressure, V is the volume of gas being considered, T is its absolute temperature, and n is the number of moles of gas.
The universal gas constant R has a value of 0.0821 L · atm/mol · K.
Comparing two equal volumes of air at the same pressure, for example, a balloon filled with cold (ambient) air and one filled with an equal amount of warm air, the Ideal Gas Law predicts that the warmer balloon will contain fewer moles of gas so the warmer balloon will be lighter because the air inside it will be less dense.
To rewrite the Ideal Gas Law in terms of density (ρ), ρ = P/RT, and for a constant pressure and gas composition, even more simply
where ρ is the air density in kg/m3, at temperature T in Kelvin. For a pressure constant at 1 atm, C is 347 K · kg/m3. (Obtained from dry air density of 1.164 kg/m3 at 20 °C and 1 atm pressure: i.e., 293 K x 1.164 kg/m3.)
Principle of Archimedes and Newton's Second Law of Motion
The Principle of Archimedes explains why this difference in temperature and gas density leads to a tendency for warm air and hot air balloons to rise. The principle states that when a body is immersed in a fluid (a liquid or a gas), an upward force is exerted on the body that is equal to the weight of the fluid the body displaces. This upward force is called buoyancy.
For a balloon containing air at the same temperature as its surroundings, the buoyancy force is balanced by the weight of the air in the balloon and there is no net force or effect. If the air is heated, it expands. It pushes out and so displaces the cooler, denser air around it. Because of this displacement, a net upward force is produced on the warm air mass, causing a tendency to rise. In a hot air balloon, the mass of warm air is trapped by the balloon's skin, allowing the difference between the buoyancy force and its weight to be harnessed if it exceeds the structural weight of the balloon and its payload.
The Principle of Archimedes can be expressed in an equation, which is more useful for engineering calculations.
From the definition of density (ρ = mass / volume = m / V), Newton's Second Law of Motion (F = ma), and for the acceleration due to gravity (a = g), the gravity force on a volume of fluid is F = (m)(a) = (ρV)(g).
Due to Archimedes' Principle and the hotter fluid being at a lower density (ρhot), the net buoyant force can be obtained from the difference Fnet = (mamb g – mhot g), where mamb is the mass of the displaced ambient air and mhot is the mass of the air inside the hot air balloon. If this net buoyant force goes entirely to lift a payload (mideal) it is,
,
Combining this equation with ρ = C / T and convenient approximations, the maximum payload of an ideal hot air balloon is
If the actual hot air balloon mass is determined by mballoon — by weighing it before it is filled with hot air — then the maximum possible payload can be determined. i.e., payloadreal < mideal – mballoon.
The Balloon Competition Ratio
This lab is a competition. NASA will judge the design's performance against the other designs in the section. The balloon competition ratio will be used to measure the performance of each design.
Payload is the number of paperclips the design can lift. Time afloat is the elapsed time from when the balloon rises to when it returns to its starting position. Cost is the cost to build the balloon.
The design will be allowed three trials.
Competition Rules
The following rules must be observed at all times during the competition. Violation of any of these rules will result in the disqualification of the balloon:
- The TA must approve the design before it can be entered in the competition
- All the materials used in the design must be purchased
- Unused materials may not be returned for credit
- The maximum balloon volume is 1 m3
- Time aloft is the elapsed time from when the balloon rises from its initial height to when it sinks to that height
- If the balloon does not rise, the time aloft is zero
- The design is limited to three trials. Indicate the number of trials performed and the ratios in the Abstract of the lab report. The lab report should also show the results for each trial including dimensions, payload, estimated balloon volume, and competition results
Design Considerations
- Which balloon shape best minimizes structural mass and effectively captures and retains warm air to fill and launch the balloon?
- How is balloon volume maximized and surface area minimized?
- Carefully consider weight, surface area, volume, material properties, and cost in the design process.
Materials and Equipment
Materials with Price List
- Drawing paper: $0.10/sheet
- Tissue wrap: $0.10/sheet
- 8 ½ x 11 paper sheets: $0.05/sheet
- Kevlar string: $0.05/30cm
- Adhesive tape: $0.03/30cm
- Plastic straws: $0
Equipment Used
- Scissors
- A glue stick
- Paper clips
- A personal heater
- A stop watch
- A thermometer
Procedure
Construct a hot air balloon using the available materials. This lab is a competition. The design with the highest ratio of payload divided by cost multiplied by time afloat will be the winner.
Sketch a preliminary design. The maximum balloon volume is 1 m3. Volume should be approximated and recorded on your lab notes.
The design must include an area near the bottom of the balloon where paperclips may be attached to add payload during the competition phase of the lab. In addition, there must be an opening that will allow hot air to enter the balloon when placed over the heater.
WARNING: Turn the heater off when not in use. Otherwise, it will become extremely hot and possibly melt the balloons.
When finished, have the sketch approved and signed by the lab TA. Construct the balloon using the materials that were selected. For the competition phase, a payload will be attached to the bottom of the balloon and it will be filled with hot air.
The lab work is now complete. Please clean up the workstation. Return all unused materials to the TA.
Assignment
Individual Lab Report
Follow the lab report guidelines laid out in the page called Specifications for Writing Your Lab Reports in the Technical Communication section of this manual. The following discussion points should be addressed in the appropriate section of the lab report:
- Discuss the importance of hot air balloons today
- Describe the rules of the competition in the Introduction. What consequences did the rules have on design decisions? In answering, use the appropriate equations
- Explain the Ideal Gas Law and the Principle of Archimedes. Include a definition and an example of each
- Describe the balloon's design. Calculate the volume of the balloon (i.e., dimensions and calculation) to show compliance with the rules. Explain the design choices. Include a discussion of the materials chosen and why. Explain the strategy for winning the competition
- Describe how the design succeeded or failed. What choices could have improved the balloon's final standing in the competition?
- Discuss and elaborate how to improve the competition ratio for this design.
- Suggest possible improvements in conducting the lab
- Include the spreadsheet with every balloon's results. Describe the results and discuss other designs in the class
Remember: Lab notes must be taken. Experimental details are easily forgotten unless written down. EG1004 Lab Notes Paper can be downloaded and printed from the EG1004 Website. Use the lab notes to write the Procedure section of the lab report. At the end of each lab, a TA will scan the lab notes and upload them to the Lab Documents section of the EG1004 Website. One point of extra credit is awarded if the lab notes are attached at the end of the lab report. Keeping careful notes is an essential component of all scientific practice.
Team PowerPoint Presentation
The following discussion points must be addressed in the appropriate section of the presentation:
- Describe the rules of the competition. What consequences did the rules have on design decisions? Use the appropriate equations in the answer.
- Since one term in the competition ratio is cost, present the cost of the balloon. Use the page How to Show Cost Data in Presentations for instructions on how to do this.
- Explain the Ideal Gas Law and the Principle of Archimedes. Include a definition and an example of each.
- Were all materials purchased used?
- Describe the balloon's design. Show the volume of the balloon (i.e., dimensions and calculation) to show compliance with the rules. Explain the design choices. Discuss the materials chosen and why they were chosen. Explain the strategy for winning the competition.
- Describe how the design succeeded or failed. What choices could have improved the balloon's final standing in the competition?
- Discuss how to improve the competition ratio.
Endnotes
- National Aeronautics and Space Administration. 2017. “Scientific Balloons.” Accessed 20 August 2017 from https://www.nasa.gov/scientificballoons
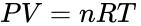
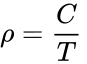
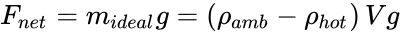 ,
,
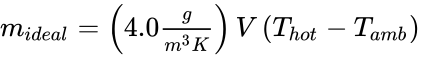
![{\displaystyle {\frac {TimeAfloat[s]}{Cost[\$]}}\times Payload\,}](https://wikimedia.org/api/rest_v1/media/math/render/png/8fd6e6d836ce7eacf86f8fbb36e5fea20ee86e6c)