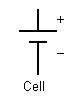Difference between revisions of "Lemon Car Competition"
| Line 194: | Line 194: | ||
<table border=1 cellspacing=0 align=center> | <table border=1 cellspacing=0 align=center> | ||
<tr><td><b>Time, τ</b></td><td><b>%Charged</b></td></tr> | <tr><td><b>Time, <i>τ</i></b></td><td><b>%Charged</b></td></tr> | ||
<tr><td>τ</td><td>63%</td></tr> | <tr><td>τ</td><td>63%</td></tr> | ||
<tr><td>2τ</td><td>86%</td></tr> | <tr><td>2τ</td><td>86%</td></tr> | ||
| Line 331: | Line 331: | ||
<li>Now connect the LED to the lemon battery in the right polarity. Place a hand | <li>Now connect the LED to the lemon battery in the right polarity. Place a hand | ||
over the LED and observe it to see if you see any light. | over the LED and observe it to see if you see any light. If it does not light | ||
connect a third lemon cell in series. Does it light up? Why/why not?</li> | connect a third lemon cell in series. Does it light up? Why/why not?</li> | ||
| Line 344: | Line 344: | ||
<li>For your convenience lemon juice and small cups will be provided for this | <li>For your convenience lemon juice and small cups will be provided for this | ||
portion of the lab. | portion of the lab. You may cut the cups to reduce weight and somehow fasten | ||
the electrodes. The lemon juice will create a better electrolytic cell than a | the electrodes. The lemon juice will create a better electrolytic cell than a | ||
lemon (which has inherent membranes that limit chemical reactions). Your lemon | lemon (which has inherent membranes that limit chemical reactions). Your lemon | ||
| Line 390: | Line 390: | ||
<p><sup>1</sup> Atkins, P.w., Beran, J.A.. <u>General Chemistry(second edition). | <p><sup>1</sup> Atkins, P.w., Beran, J.A.. <u>General Chemistry(second edition). | ||
</u> | </u> American Books, 1989</p> | ||
<p><sup>2</sup> Wikipedia. 16 Aug. 2005 | <p><sup>2</sup> Wikipedia. 16 Aug. 2005 | ||
{http://en.wikipedia.org/wiki/Electrode}</p> | {http://en.wikipedia.org/wiki/Electrode}</p> | ||
Revision as of 21:03, 20 August 2005
Lab 11: Lemon Car
1 Objectives
The experimental objective of this lab is to study the principles of a reduction oxidation reaction, and to learn about how a capacitor stores energy.
In order to learn how battery cells, capacitors and different reducing agents work, we will construct a lemon citrus cell and use the cell to power two different devices.
2 Overview
Chemistry behind a Citrus Cell
A battery is a device that is usually made up of many individual cells. In this experiment we will be creating a citrus cell. A battery cell is a cell that creates a voltage across a terminal of 2 dissimilar metals. This is done through a chemical half reaction. A citrus cell is one that does this using a citrus juice as the electrolytic solution. By using 2 different types of metals we have 2 different reaction potentials which directly affect the voltage produced with each cell. By linking multiple cells together in series we produce a battery.
Before learning the concept behind a battery cell, there are two properties that should be understood: electronegativity and ionization energy:
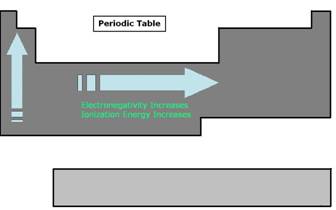
- Electronegativity in an element is a measure of the element’s capability to attract another’s electrons. The higher degree of electronegativity an element has the more power it has to pull away electrons from a lesser electronegative element. There is an obvious trend in the periodic table for this particular property. Electronegativity generally increases from left to right and from bottom to top. As a result the elements around cesium have very low electronegativity and elements around fluorine have the highest.
- Ionization energy is the amount of energy required to remove an electron from an atom to form a cation. A cation is ion or group of ions having a positive charge in electrolysis. This property also has a trend in the periodic table. It generally increases from left to right and from bottom to top.
Figure 1: Periodic Table Overview
The electronegativity and ionization energy values for the metals used in the lab are provided in the table below:
| Metal | Electronegativity (Pauling Scale) |
Ionization Energy (kJ/mole) |
| Magnesium | 1.31 | 738 |
| Nickel | 1.91 | 736 |
| Copper | 1.90 | 745 |
| Zinc/td> | 1.65 | 904 |
| Aluminum | 1.61 | 577 |
These two properties give the metals their reactive traits.
The reaction that takes place in the lemon cell is called a redox reaction. This reaction can be simplified into to two parts and they are called oxidation and reduction. Oxidation happens when there is an electron loss and reduction is when there is an electron gain. Oxidation and reduction can be shown in a half-reaction, which clearly shows the electron transfer. Take for example the redox reaction between magnesium and oxygen.
2Mg(s) + O2(g) → 2MgO2(s)
When magnesium reacts with oxygen, magnesium loses electrons making it oxidized:
Mg(s) → Mg2 + (s) + 2e-
At the same time, oxygen gains those electrons making oxygen reduced.
O2(g) + 4e- → 2O2-(g)
These are the half-reactions of the magnesium and oxygen redox reaction. Be aware that the electrons in the half-reactions are not given a state, that is because they are being transferred and don’t have a definite state. If these reactions were separate then the electrons would have to travel through a wire or some medium to get to the other side, causing the two half-reactions to have a potential between each other. This potential is the voltage between the two half-reactions.
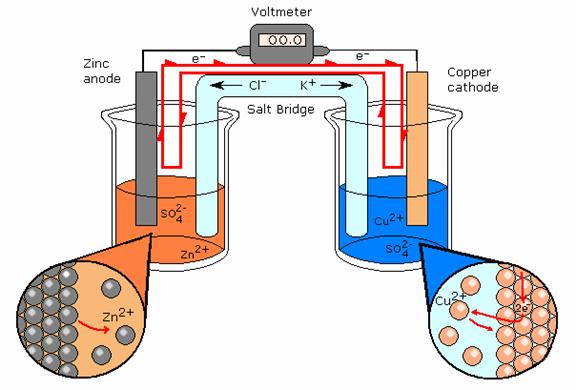
An electrochemical cell is where two electrodes are in contact with an electrolyte. The electrodes are conductors (usually metallic) used to form a contact with a nonmetallic part of a circuit such as an electrolyte.2 The electrolyte provides an abundance of ions to the reaction. The electrode at which oxidation occurs is called the anode and is the negative part of the cell. The electrode at which reduction occurs is called the cathode and is the positive part of the cell.
Based on McGraw Hill Drawing
Electrochemical Cell- Daniell’s Cell
A type of electrochemical cell is shown above. The path of electron travel is shown clearly by the red line. This electrochemical cell is more specifically called a Daniell’s Cell. There is a salt bridge separating the half-reactions. This salt bridge is present to provide each side with ions. Oxidation of Zn in the galvanic cell produces Zn+ ions, also reduction of Cu- ions. Without the salt bridge to connect the half-reactions and bring ions the reactions would not continue. The ions from the half-reaction would plate the other electrode, and the reaction would slowly stop when the ions have finished plating the opposite electrode. The reaction ‘poisons’ itself.
Reference: McGraw Hill
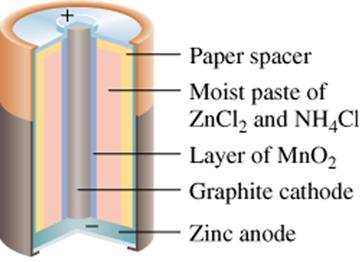
The concept of a Daniell’s Cell when applied to a commercial battery is called the Leclanché cell. In a Leclanché cell there are two electrodes and an electrolyte and they are separated using a porous material. This material only lets the ions pass through, therefore preventing direct interaction of the two half-reactions.1
Electrical Components
In electrical engineering different electrical components are represented by different symbols. Below a few of them are shown:
A cell is a single unit for the conversion of chemical energy to electrical energy. A battery is comprised of multiple cells linked together.
A capacitor is an electrical device used to store charge temporarily. Different capacitors have different polarities.
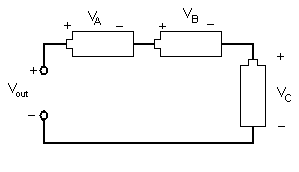
Different combinations of electrical components allow engineers to design different devices. Resistors, inductors, and capacitors can be arranged in three different ways. In a series circuit, the element's conductors are connected end to end. The current in a series circuit remains the same in all the electrical elements. In a serial circuit, as shown in Figure 9.4, the sum of the voltages across each element is equal to the voltage of the power source ( Vout = VA + VB + VC).
Figure 9.4: A series circuit
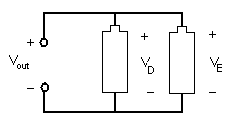
In a parallel circuit, as shown in Figure 9.5, the element's conductors are connected at opposing ends. The current that is supplied by the voltage source equals the current that flows though elements D and E . The voltage across the elements that are parallel is the same (V out = VD = VE)).
Figure 9.5: A parallel circuit
Capacitors are devices that are used to store charge. They can be used in replace of a battery but they operate very differently from one. A capacitor is charged by a voltage source exponentially. Shown by the formula
Equation 1: The 98% charged time constant
The time constant is the standard unit of time for a capacitor to charge to 68%. However capacitors do not charge linearly, so for each subsequent interval of τ the capacitor will accumulate less energy. See the following chart.
| Time, τ | %Charged |
| τ | 63% |
| 2τ | 86% |
| 3τ | 95% |
| 4τ | 98% |
| 5τ | 99% |
Chart 1: Time versus % charged.
When a capacitor is fully charged, it does not matter what voltage it was when it was charged. It will output the voltage the capacitor is rated for when it is fully charged.
The motor provided is a 9V motor that needs a minimum of 2.5 volts to operate.
A motor with no load draws 9 milliamps; a stalled motor draws well over 350 milliamps. By increasing the voltage provided to the motor you can increase the motor speed. By increasing current you increase the torque.
3 Your Assignment
Team PowerPoint Presentation and Individual Lab Report
- Describe the electrochemical cell in your introduction. What consequences did the electrode voltages have for your design decisions? Use appropriate part 1 experimental values.
- What factors did you consider in designing your Lemon-Powered Car? Did you use any of the background information?
- What was the competition ratio for your design?
- What important design characteristics should a winning Lemon-Powered Car include to achieve the highest possible design ratio?
- Describe how your power source design succeeded or failed.
- Discuss design improvements. How would you optimize the design (i.e. improve the ratio) based on experience?
- Describe the concept behind the lemon battery.
4 Materials and Equipment
- Lemons
- Lemon Juice
- Magnesium
- Zinc
- Copper
- Nickel
- Aluminum
- 3 Alligator cable set
- 1 Farad 2.5V Capacitor
- Standard Lego Car Chassis
- Lego to Alligator Cable Clip Connector
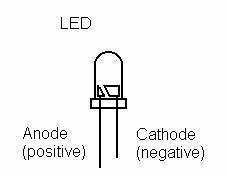
- Light Emitting Diode (LED)
- Small cups
- Scissors
- Tape
- DMM (Digital Multi-meter)
- Large Plates
Warning: Magnesium is a highly reactive metal. Use carefully and only as described in these instructions.
5 Procedure
Part 1
Remember: You are required to take notes. Experimental details are easily forgotten unless written down. You should keep a laboratory notebook for this purpose. Use your lab notes to write the Procedure section of your lab report. You must attach a copy of your lab notes to the WC copy of your lab report. Keeping careful notes is an essential component of all scientific practice.
- Obtain two different samples of metals from your TA (two each). TA’s will select different metal combinations for the students.
- Take the lemon and squeeze/roll it so that the lemon juice inside the pulp is released, without puncturing the lemon.
- Insert one of the metals into the lemon.
- Half a centimeter away insert the other metal piece parallel to the first, so that they are close but do not touch.
- Connect one end of the first alligator cable set to one of the metal pieces and one end of the second alligator cable set to the other metal. These will be the electrodes of the cell.
- Connect the other ends of the alligator cable sets to the positive and negative ends of the digital multi-meter and measure the voltage. Record the value in units of volts. (Note: If it is negative then the polarity is switched, switch the alligator clips to connect to the opposite sides of the DMM)
- The metal strip attached to the positive end of the digital multi-meter (red wire) is the anode (+) and the other (black wire) is the cathode (-).
- Record the values and present them to your TA. The TA will generate a spreadsheet with the amount voltage produced by each metal combination for all teams.
- Remove the electrodes to stop the chemical reaction. Wipe them clean with a paper towel. Discard the paper towel in the trash can.
- Repeat steps 2-4 in part 1 to create a second citrus cell.
- Insert the metals into the first lemon to reactivate the first citrus cell.
- Connect the first lemon’s anode to the second lemon’s cathode using the alligator cable set. Now you have created a lemon battery.
- Measure the voltage in the lemon battery using the digital multi-meter and record it. Following the formula for linking batteries in series, the voltage should be doubled. If it is lower try taking the electrodes out and plunging them into a different part of the lemon.
- Now connect the LED to the lemon battery in the right polarity. Place a hand over the LED and observe it to see if you see any light. If it does not light connect a third lemon cell in series. Does it light up? Why/why not?
- Clean/return all electrodes.
- Assess your materials and consider the data established from part 1. Choose your materials for your lemon juice battery car design, keeping in mind the limit of four cells per battery (Note: Keep in mind the Competition Ratio). Make sure you take notes and make preliminary sketches during this process.
- For your convenience lemon juice and small cups will be provided for this portion of the lab. You may cut the cups to reduce weight and somehow fasten the electrodes. The lemon juice will create a better electrolytic cell than a lemon (which has inherent membranes that limit chemical reactions). Your lemon car can be powered by either a lemon battery or a lemon battery charged capacitor.
- You will be given a standard chassis for your lemon car. You may modify it any way you deem fit. Sketch your design in pencil using the graph paper provided on the EG website http://eg.poly.edu. Label your drawings clearly. Prepare a price-list for your lemon powered car based on the materials you have chosen. Use the results your class determined from Part 1 of the experiment. Have your TA sign the sketches and the price-list.
- Your TA will provide the materials needed for your design. If you decide to modify your design during the construction of your insulating container, note the changes and describe the reasons for them. If the modifications required more materials to be used, make sure you update your price list and your TA approves it.
Warning: magnesium left in the lemon juice dissolves.
Part 2
Part 3: Lemon Powered Car Design
6 Competition
Discharge the capacitor by connecting the alligator cable set to both terminals. Charge the capacitor to the setup from part 3 for at least 15 minutes. (Warning: don’t discharge capacitor by accident)
The performance of your lemon powered car will be measured by the following formula:
The student will position the lemon powered car and connect thealligator clips to the lemon car when requested by the TA. The TA will record the test data after 60 seconds or when the car stops moving. The TA will then calculate the competition ratio. The tabulation for the whole class will be provided.
7 Data Analysis
Your lab work is now complete. Please clean up your workstation. Return all unused materials to your TA. Refer to section Your Assignment for the instructions you need to prepare your lab report.
Footnotes
1 Atkins, P.w., Beran, J.A.. General Chemistry(second edition). American Books, 1989
2 Wikipedia. 16 Aug. 2005 {http://en.wikipedia.org/wiki/Electrode}