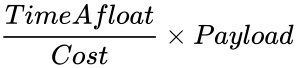Hot Air Balloon Competition
Objectives
The objective of this lab is to design and build a hot air balloon. This is a competition lab. Your design should maximize the amount of non-structural weight (payload) that your balloon can lift and the time it can spend aloft, while minimizing the balloon's structural weight and its cost. In designing your balloon you may wish to make use of several concepts from physics, including the Ideal Gas Law, and the Principle of Archimedes.
Overview
At Versailles on September 19, 1783, two French brothers, Joseph-Michel and Jacques-Étienne Montgolfier, loaded a sheep, a rooster, and a duck into the basket of their hot air balloon and untethered the ship for the entertainment of the King of France, Louis XVI. They had gotten the idea from watching hot embers rise and float above a fire. Later, in November 1783, they launched the first manned balloon flight[1].
Hot air balloons are an example of lighter-than-air aircraft. They are widely used for recreation and advertising. Another example of this type of aircraft is the dirigible, which consists of a rigid steel frame with bags of light gas inside it, causing it to lift. Because of the weight of the frame, an extremely light gas such as hydrogen had to be used, which is extremely flammable. This led to safety problems, so dirigibles are no longer in service. A blimp is a lighter-than-air aircraft which is essentially a big helium balloon with engines attached. It does not have a structural frame like a dirigible, and got its name from the sound it makes when you hit it with something. An aerostat is a blimp that doesn't have engines, but is attached by a tether (rope) to the ground. Aerostats are frequently used for advertising or finding a location. The person trying to find the location just looks for the aerostat in the sky attached to the location and travels to it. Aerostats are also used as weather stations and radar platforms since they are so far up. Building a structure that tall would probably be impossible.
Background Concepts
The fundamental concepts are the Ideal Gas Law, gas density, the Principle of Archimedes, and Newton's Second Law of Motion.
Ideal Gas Law and Gas Density
Usually, when an object is heated it expands. The same amount of mass occupies a larger volume and therefore the density of the object decreases. For gases, this is especially important because the expansion can be quite pronounced, and a mass of warm air is considerably less dense than an equal mass of cold air.
This idea can be expressed quantitatively by using the Ideal Gas Law to describe the behavior of air. This law states that
where P is the gas pressure, V is the volume of gas being considered, T is its absolute temperature and n is the number of moles of gas. The universal gas constant R has a value of 0.0821 L atm/mol K.
Comparing two equal volumes of air at the same pressure (say a balloon filled with cold [ambient] air and one filled with an equal amount of warm air), the Ideal Gas Law predicts that the warmer one will contain fewer moles of gas, so the warmer balloon will be lighter because the air inside it will be less dense.
Actually it is most convenient to rewrite the ideal gas law in terms of density (ρ), ρ = P / (RT), and for a constant pressure and gas composition, even more simply
where ρ is the air density in kg/m3, at temperature T in Kelvin. For a pressure constant at 1 atm, C is 347 K kg/m3. (Obtained from dry air density of 1.164 kg/m3 at 20 °C and 1 atm pressure: i.e., 293 K x 1.164 kg/m3.)
Principle of Archimedes and Newton's Second Law of Motion
But why does this difference in temperature and gas density lead to a tendency for warm air (and hot air balloons) to rise? The Principle of Archimedes provides an explanation. The principle states that when a body is immersed in a fluid (a liquid or a gas), an upward force is exerted on the body equal to the weight of the fluid the body displaces. This upward force is called buoyancy.
For a balloon containing air at the same temperature as its surroundings, the buoyancy force is balanced by the weight of the air in the balloon and there is no net force or effect. If the air is heated however, it expands. It pushes out and so displaces the cooler ambient air. Because of the displacement of the cooler, denser air, a net upward force is produced on the warm air mass, causing a tendency to rise. In our hot air balloon, the mass of warm air is trapped by the balloon's skin, allowing the difference between its buoyancy force and its weight to be harnessed if it exceeds the structural weight of the balloon and its payload.
Okay — let's put the Principle of Archimedes into a more useful form for engineering calculations (an equation).
From the definition of density (ρ = mass / volume = m / V), Newton's Second Law of Motion (F = ma), and for the acceleration due to gravity (a = g), the gravity force on a volume of fluid is F = (m)(a) = (ρV)(g).
Due to Archimedes' Principle and the hotter fluid being at a lower density (ρhot), the net buoyant force can be obtained from the difference Fnet = (mamb g – mhot g), where mamb is the mass of the displaced ambient air and mhot is the mass of the air inside the hot air balloon. If this net buoyant force goes entirely to lift a payload (mideal) we obtain,
,
Finally, combining the above equation with ρ = C / T and convenient approximations, the maximum payload of an ideal hot air balloon is
Incidentally, if the actual hot air balloon mass is determined by mballoon — by weighing it before it is filled with hot air — then the maximum possible payload can be determined. i.e., payloadreal < mideal – mballoon.
The Balloon Competition Ratio
Think carefully about your design. What balloon shape will you use to minimize structural mass and to effectively capture and retain warm air as you try to fill and launch your balloon? How will you maximize balloon volume and minimize surface area? Cost is also an important concern. Carefully consider weight, surface area, volume, material properties, and expense in your design process.
This lab is a competition. Your design's performance will be judged against the other designs in your section. The Balloon Competition Ratio will be used to measure the performance of each team
You will be allowed three trials.
The competition ratio does not include the hot-to-ambient air temperature difference, however, the data should be provided in your table of results — to help explain the results.
Actually, the temperature measurement is a new addition to this lab (effective Fall 2011) and it remains to be seen if the values vary sufficiently to merit adjusting the competition ratio (by dividing) by quantity Thot – Tamb, or if reasonably precise temperature measurements are even practical.
Your Assignment
*BONUS* Individual Lab Report
Follow the lab report guidelines laid out in the page called Specifications for Writing Your Lab Reports in the Technical Communication section of this manual. As you write, the following discussion points should be addressed in the appropriate section of your lab report:
- Describe the rules of the competition in your introduction. What consequences did the rules have for your design decisions? Use the appropriate equations in your answer.
- Explain the Ideal Gas Law and the Principle of Archimedes. Make sure you include a definition and an example of each.
- Discuss minimal design. Did you use all the materials you purchased? Describe the importance of minimal design and explain how you employed it in your design.
- Describe your balloon's design. Show the volume of your balloon (i.e., dimensions and calculation) to show that you stayed within the rules. Explain the choices you made. Make sure you include a discussion of the materials you chose and why. Explain your team’s strategy for winning the competition.
- Describe how your design succeeded or failed. What choices could you have made to improve your final standing in the competition?
- Discuss how you would improve the competition ratio.
Team PowerPoint Presentation
The following discussion points are to be addressed in the appropriate section of your presentation:
- Describe the rules of the competition. What consequences did the rules have for your design decisions? Use the appropriate equations in your answer.
- Since one term in the competition ratio is cost, present the cost of your balloon. Use the page How to Show Cost Data in Presentations for instructions on how to do this.
- Explain the Ideal Gas Law and The Principle of Archimedes. Make sure you include a definition and an example of each.
- Discuss minimal design. Did you use all the materials you purchased? Describe the importance of minimal design and explain how you employed it in your design.
- Describe your balloon's design. Show the volume of your balloon (i.e., dimensions and calculation) to show that you stayed within the rules. Explain the choices you made. Make sure you include a discussion of the materials you chose and why. Explain your team's strategy for winning the competition.
- Describe how your design succeeded or failed. What choices could you have made to improve your final standing in the competition?
- Discuss how you would improve the competition ratio.
Competition Rules
The following rules must be observed at all times during the competition. Violation of any of these rules will result in the disqualification of your balloon:
- The TA must approve your design before it can be entered in the competition.
- All the materials you use in your design must be purchased.
- You may not return unused materials for credit.
- The Maximum Balloon volume is 1 m3.
- Time aloft is defined to be the elapsed time from when the balloon rises from its initial height to when it sinks to that height.
- If the balloon does not rise, the time aloft is zero.
- You are limited to three trials. You should indicate the number of trials you actually used in the abstract of your lab report. Your lab report should also show the results for each trial in terms of dimensions, payload, heater and ambient air temperatures, and competition ratio results.
Materials and Equipment
Materials with Price List
- Drawing Paper: $0.10/sheet
- Tissue Wrap: $0.10/sheet
- 8 ½ x 11 Paper Sheets: $0.05/sheet
- Kevlar String: $0.05/foot
- Adhesive Tape: $0.03/foot
- Plastic Straws: $0
Equipment Used
- Scissors
- Glue Stick
- Paper Clips
- Personal Heater
- Stop Watch
- Themometer
Procedure
Construct a hot air balloon using household materials. This lab is a competition. The team with the highest ratio of payload, divided by cost, multiplied by time aloft, will be declared the winner.
First, sketch your preliminary design. The maximum balloon volume is 1 m3.
Note: Your design must include an area near the bottom of the balloon where paper clips may be attached in order to add weight during the competition phase of the lab. In addition, there must be an opening that will allow the balloon to be placed over the heater.
WARNING: Turn the heater off when you're done using it. Otherwise, it will become extremely hot and possibly melt the balloons.
When you are finished, have your sketch approved and signed by the lab TA. Construct your balloon using the materials you have selected. For the competition phase, your team will attach weight to the bottom of your balloon. Obtain the ambient temperature in the room, and measure the hot air temperature leaving the heater after the temperature reading is reasonably constant, and then fill your balloon with hot air. The winners are determined by the Balloon Competition Ratio.
Note: For this competition, the bonuses will be awarded to the PowerPoint Presentations since there is no full lab report for this lab.
Your lab work is now complete. Please clean up your workstation. Return all unused materials to your TA.
Endnotes
- ^ "Montgolfier, Joseph-Michel and Jacques-Étienne" Britannica Student Encyclopedia from Encyclopædia Britannica Online. http://www.search.eb.com.databases.poly.edu/ebi/article?tocId=9275924 [Accessed November 3, 2004].
| ||||||||
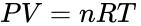
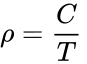
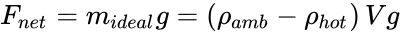 ,
,
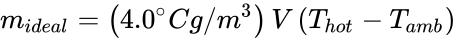
![{\displaystyle {\frac {TimeAfloat[s]}{Cost[\$]}}\times Payload\,}](https://wikimedia.org/api/rest_v1/media/math/render/png/8fd6e6d836ce7eacf86f8fbb36e5fea20ee86e6c)